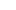Sterilization Indicators Consumables Manufacturers
Eray MedTech sterilization indicators are critical tools for validating the effectiveness of sterilization processes, ensuring equipment, instruments, or materials achieve a sterile state. They are widely used in healthcare, laboratories, and industrial settings.
With a building area of 20,310 square metres, the company has a class 100,000 purified production workshop, a class 10,000 microbiology testing room, a local class 100 physical and chemical laboratory, and a standardised storage system for raw materials and finished products.
Since the initial batch of products were launched in 2013, Eray has continuously enriched its product categories. Our products have covered protective masks, nursing consumables, sensory control consumables, surgical instruments, providing safe, efficient and environmentally friendly disposable medical solutions for medical institutions worldwide.
As a professional OEM Sterilization Indicators Consumables Manufacturers and ODM Sterilization Indicators Consumables Factory, The company has passed ISO 13485 and other quality system certifications, and some of its products have obtained CE certification and FDA filing permits, and has established long-term cooperative relationships with many domestic and foreign medical institutions and distributors.
-
Feb 27. 2026
Can Optical Trocars Reduce the Risk of Organ Injury?Optical Trocars Significantly Reduce Organ Injury Risk Yes, optical trocars can significantly reduce the risk of organ injury during laparoscopic surgery by allowing surgeons to visualize tissue layers in real time as the instrument passes through the abdominal wall. Unlike blin...
Read More -
Feb 20. 2026
What is an Optical Trocar and Why is it Essential in Medical Procedures?What is an Optical Trocar and Why is it Essential in Medical Procedures? An optical trocar is a specialized surgical instrument used primarily in minimally invasive procedures. It combines the functions of a trocar and a visual scope, allowing surgeons to create an access point ...
Read More -
Feb 13. 2026
What Are the Common Problems With Medical Trocars and How to Avoid Them?What Are the Most Common Problems With Medical Trocars? The most common problems with medical trocars include insertion injuries, gas leakage, dull blades, instability, and contamination risks. These issues can compromise surgical safety, increase operating time, and raise posto...
Read More
The effectiveness of the sterilization process is directly related to patient safety and the reliability of laboratory results. Sterilization indicators, as key tools for verifying sterilization effectiveness, provide conclusive evidence of aseptic operation through scientific methods and become an indispensable component of infection control systems.
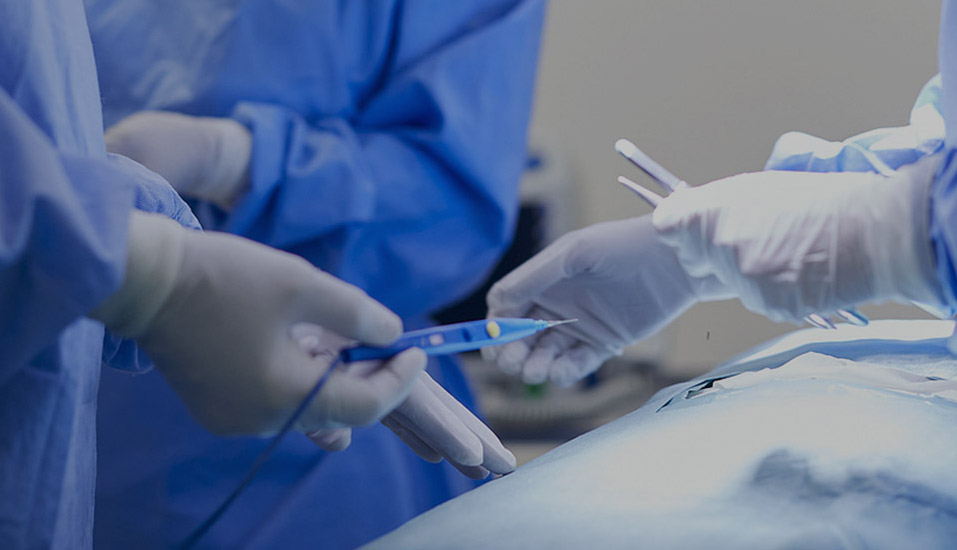
Sterilization indicators operate based on their sensitivity to key sterilization parameters. Chemical indicators use an irreversible color change reaction of specific inks or dyes at specific temperatures, times, or chemical concentrations to visually indicate whether sterilization conditions have been met. Biological indicators use highly heat-resistant Bacillus stearothermophilus spores as challenge microorganisms. Their survival is monitored after sterilization through post-sterilization culture, providing the most direct proof of sterilization effectiveness. New electronic indicators use precision sensors to record physical parameters such as temperature, pressure, and steam saturation in real time, generating digital verification reports. These complementary approaches form a multi-layered sterilization verification system. Modern sterilization indicators offer distinct technical features: They can detect temperature deviations of ±1°C and time errors of ±5% with sensitivity. Their response range covers the typical sterilization temperature range of 121°C to 134°C. Some products also offer specialized models for low-temperature sterilization, such as ethylene oxide and hydrogen peroxide.
Proper use of sterilization indicators requires careful consideration of several key points: Select the appropriate indicator type based on the sterilization method (steam, dry heat, chemical, etc.); ensure placement is representative, typically in the most challenging area of the sterilization chamber; avoid contact with liquids or sharp instruments, which could damage the markings; and ensure that biological indicators are incubated according to regulations after use, typically at 56-60°C for 24-48 hours. All indicators should be stored in a dry, dark environment, and their expiration dates must be carefully monitored. Importantly, any abnormal indication should be considered a sterilization failure, requiring immediate cessation of use of the relevant batch and investigation of the cause.
Maintenance of sterilization indicators is crucial for ensuring reliable sterilization monitoring, and strict management practices are essential. During daily use, indicators should be stored in a cool, dry place, away from direct sunlight and high temperatures and humidity. The recommended temperature is 15-25°C, and the relative humidity should not exceed 60%. Different types of indicators should be stored separately. Chemical indicator cards should be sealed to prevent moisture, and biological indicators should be refrigerated and used within their expiration dates. Before use, the integrity of the packaging should be inspected. For chemical indicators, the color should be checked for normality. For biological indicators, the culture medium should be confirmed to be free of desiccation or contamination. During each sterilization cycle, the indicator should be placed in the most challenging location within the sterilization chamber, typically in the center of the sterilized items or near the drain. Read the results promptly after use, read the chemical indicator cards within the specified time, and maintain a constant incubator temperature of 56-60°C for biological indicators. Regular quality verification of indicators is essential, with each new batch undergoing performance testing. For biological indicators, positive controls should be used to verify incubation results. Establish a comprehensive record-keeping system, detailing the use date, sterilization parameters, and monitoring results for each batch of indicators, with a retention period of at least three years. Expired indicators must be discarded according to prescribed procedures and must not be used. Operators must also receive regular professional training to ensure they are proficient in the use of various indicators and the interpretation of results. Through standardized maintenance and management, sterilization monitoring data can be ensured to be accurate and reliable, providing a strong guarantee for medical safety.



 English
English Español
Español Français
Français






























 CONTACT US
CONTACT US