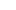
With a building area of 20,310 square metres, the company has a class 100,000 purified production workshop, a class 10,000 microbiology testing room, a local class 100 physical and chemical laboratory, and a standardised storage system for raw materials and finished products.
Since the initial batch of products were launched in 2013, Eray has continuously enriched its product categories. Our products have covered protective masks, nursing consumables, sensory control consumables, surgical instruments, providing safe, efficient and environmentally friendly disposable medical solutions for medical institutions worldwide.
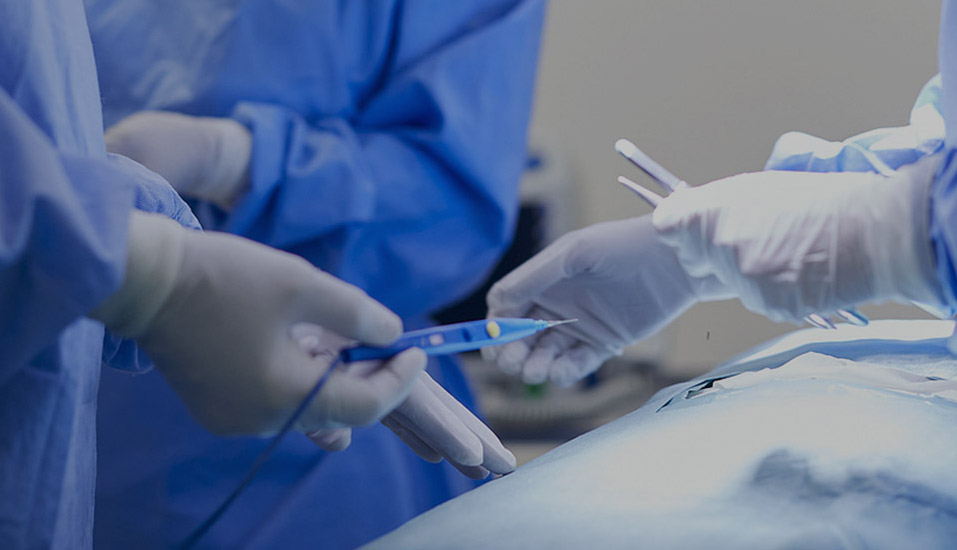
As a professional OEM Steam Sterilizers Suppliers and ODM Steam Sterilizers Factory, The company has passed ISO 13485 and other quality system certifications, and some of its products have obtained CE certification and FDA filing permits, and has established long-term cooperative relationships with many domestic and foreign medical institutions and distributors.
-
Feb 27. 2026
Can Optical Trocars Reduce the Risk of Organ Injury?Optical Trocars Significantly Reduce Organ Injury Risk Yes, optical trocars can significantly reduce the risk of organ injury during laparoscopic surgery by allowing surgeons to visualize tissue layers in real time as the instrument passes through the abdominal wall. Unlike blin...
Read More -
Feb 20. 2026
What is an Optical Trocar and Why is it Essential in Medical Procedures?What is an Optical Trocar and Why is it Essential in Medical Procedures? An optical trocar is a specialized surgical instrument used primarily in minimally invasive procedures. It combines the functions of a trocar and a visual scope, allowing surgeons to create an access point ...
Read More -
Feb 13. 2026
What Are the Common Problems With Medical Trocars and How to Avoid Them?What Are the Most Common Problems With Medical Trocars? The most common problems with medical trocars include insertion injuries, gas leakage, dull blades, instability, and contamination risks. These issues can compromise surgical safety, increase operating time, and raise posto...
Read More
Steam sterilizers are specialized equipment that utilize high-temperature, high-pressure saturated steam for sterilization. They play a vital role in the medical, laboratory, pharmaceutical, and food industries. Through efficient moist heat sterilization, they ensure the sterility of items such as medical devices, laboratory supplies, pharmaceutical packaging, and food containers, ensuring safe use.
The core operating principle of a steam sterilizer is to generate high-temperature, high-pressure saturated steam by heating water. The steam sterilizer typically operates at temperatures between 121°C and 134°C and pressures between 100 and 200 kPa. Under these conditions, the protein structure of microorganisms undergoes irreversible denaturation, destroying their enzyme systems and achieving thorough sterilization. Furthermore, the high-pressure steam can penetrate deep into the structure of the item, ensuring comprehensive sterilization, effectively killing even heat-resistant bacterial spores.
Steam sterilizers can be categorized into several main types depending on the application requirements. Gravity-displacement sterilizers rely on the natural downward flow of cool air and are suitable for sterilizing items such as general instruments and culture media. Pre-vacuum sterilizers use a pre-vacuum process followed by steam injection to improve sterilization efficiency, making them particularly suitable for processing complex, porous items such as surgical kits. Pulsating vacuum sterilizers, on the other hand, use multiple cycles of vacuuming and steam injection to ensure thorough air removal, making them suitable for applications requiring the highest sterilization standards, such as implants.
Steam sterilizer operation requires strict adherence to specifications. Items must be properly loaded to ensure uniform steam penetration, and packaging materials must be breathable, such as specialized sterilization wrapping paper or non-woven fabric. Liquids must be slowly vented after sterilization to prevent violent boiling. To ensure sterilization effectiveness, quality control measures typically combine physical monitoring (recording temperature, pressure, and time), chemical monitoring (color change of the sterilization indicator), and biological monitoring (verification using Bacillus stearothermophilus spores). The advantages of steam sterilizers lie in their efficiency, reliability, and cost-effectiveness, making them suitable for sterilizing large quantities of items. However, they also have limitations, such as being unsuitable for materials that are sensitive to heat or moisture, such as certain plastics or precision electronic devices. Therefore, when choosing a sterilization method, it is necessary to make a reasonable match based on the characteristics of the items and industry standards.
Steam sterilizer maintenance is crucial for ensuring the quality of medical sterilization, requiring a comprehensive maintenance system. During daily use, drain the sterilization chamber of condensate after each run, wipe the chamber walls and door seals with a soft cloth to remove residual water stains and dirt. Weekly, focus on cleaning the drain filter and steam generator, using a dedicated descaling agent to dissolve scale and prevent pipe blockages that could affect steam quality. Monthly calibration of key components such as pressure gauges and temperature sensors is required to ensure measurement accuracy meets sterilization parameters. The operating status of the safety valve and vacuum pump should also be checked, and the door seals tested for airtightness. Quarterly, sterilization effectiveness should be verified using biological indicators, using standard strains such as Bacillus stearothermophilus to test the reliability of the sterilization procedure.
During equipment maintenance, special attention should be paid to water quality management. Purified or distilled water must be used, and water conductivity should be regularly tested to prevent scale buildup that could damage the heating elements. Instrument racks and loading carts within the sterilization chamber should be disassembled and cleaned monthly, inspected for deformation or rust. Before daily operation, check steam saturation to ensure it is free of excessive impurities. After sterilization, drain any remaining water from the boiler promptly. Maintain a complete maintenance log, detailing each maintenance session, troubleshooting, and component replacement. Operators must receive professional training and be proficient in equipment operation procedures and common troubleshooting methods. During extended periods of downtime, drain all pipes thoroughly, perform rust prevention measures, and conduct a comprehensive inspection before reactivation.



 English
English Español
Español Français
Français























 CONTACT US
CONTACT US