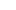Laboratory Sterilizer Suppliers
information to be updated
With a building area of 20,310 square metres, the company has a class 100,000 purified production workshop, a class 10,000 microbiology testing room, a local class 100 physical and chemical laboratory, and a standardised storage system for raw materials and finished products.
Since the initial batch of products were launched in 2013, Eray has continuously enriched its product categories. Our products have covered protective masks, nursing consumables, sensory control consumables, surgical instruments, providing safe, efficient and environmentally friendly disposable medical solutions for medical institutions worldwide.
As a professional OEM Laboratory Sterilizer Suppliers and ODM Pharmaceutical Sterilizer Factory, The company has passed ISO 13485 and other quality system certifications, and some of its products have obtained CE certification and FDA filing permits, and has established long-term cooperative relationships with many domestic and foreign medical institutions and distributors.
-
Feb 27. 2026
Can Optical Trocars Reduce the Risk of Organ Injury?Optical Trocars Significantly Reduce Organ Injury Risk Yes, optical trocars can significantly reduce the risk of organ injury during laparoscopic surgery by allowing surgeons to visualize tissue layers in real time as the instrument passes through the abdominal wall. Unlike blin...
Read More -
Feb 20. 2026
What is an Optical Trocar and Why is it Essential in Medical Procedures?What is an Optical Trocar and Why is it Essential in Medical Procedures? An optical trocar is a specialized surgical instrument used primarily in minimally invasive procedures. It combines the functions of a trocar and a visual scope, allowing surgeons to create an access point ...
Read More -
Feb 13. 2026
What Are the Common Problems With Medical Trocars and How to Avoid Them?What Are the Most Common Problems With Medical Trocars? The most common problems with medical trocars include insertion injuries, gas leakage, dull blades, instability, and contamination risks. These issues can compromise surgical safety, increase operating time, and raise posto...
Read More
In the pharmaceutical production process, sterilization is a critical step in ensuring product safety and efficacy. Pharmaceutical sterilizers, as core equipment in sterile pharmaceutical production, use high temperatures, high pressures, or other sterilization methods to thoroughly eliminate microorganisms in pharmaceuticals, packaging materials, and production equipment, ensuring safe and reliable medications for patients. From injectables to biologics, from medical devices to pharmaceutical excipients, pharmaceutical sterilizers play an irreplaceable role in every aspect of the pharmaceutical industry.
The core role of pharmaceutical sterilizers is to provide reliable sterility assurance. Microbial contamination of pharmaceuticals not only reduces efficacy but can also lead to serious medical accidents. Pharmaceutical sterilizers precisely control sterilization parameters such as temperature, pressure, and time to ensure that the sterilization process meets standards. Common sterilization methods include moist heat sterilization (saturated steam), dry heat sterilization, and ethylene oxide sterilization. Moist heat sterilization is the most commonly used sterilization method for injectable and infusion products due to its high efficiency and cost-effectiveness.
Pharmaceutical sterilizers are constructed of high-quality stainless steel, ensuring long-term stable operation under high-temperature and high-pressure environments. The intelligent control system monitors and records key parameters such as temperature, pressure, and F0 value (an indicator of microbial killing effectiveness) during the sterilization process in real time. This data is traceable and complies with FDA 21 CFR Part 11 electronic record requirements. Furthermore, the sterilizer's internal design prioritizes uniform heat distribution. Through strategically placed steam nozzles or fan circulation systems, the temperature difference within the sterilization chamber does not exceed ±1°C, avoiding sterilization blind spots. For specialized dosage forms, such as lyophilized powder injections, through-the-wall sterilizers can also be installed to achieve aseptic transfer and prevent secondary contamination. Pharmaceutical sterilizers are not just standalone devices; they are a crucial component of the aseptic production system for pharmaceuticals. In modern pharmaceutical workshops, sterilizers are often integrated with cleaning machines, filling machines, isolators, and other equipment to form a complete aseptic production line.
Pharmaceutical sterilizer maintenance is critical to ensuring pharmaceutical production safety and reliable sterilization results, requiring a rigorous, standardized maintenance process. Condensate within the sterilization chamber should be drained immediately after daily use. The interior of the chamber, door seals, and storage shelves should be wiped clean with a dedicated dust-free cloth, paying particular attention to removing residual water stains and drug particles. Weekly cleaning of the steam generator and steam trap is essential. A pharmaceutical-grade descaling agent should be used to dissolve scale deposits in the piping. After completion, the system should be repeatedly flushed with injection water until the conductivity meets the specified standard. Pressure gauges, temperature sensors, and safety valves should be calibrated and tested monthly to ensure measurement accuracy meets GMP standards. The vacuum pump oil level and quality should also be checked, and the dedicated vacuum pump oil should be replaced if necessary.
Quality control of the sterilization medium is crucial. Pure steam or hydrogen peroxide that meets pharmacopoeial standards must be used, and its purity and saturation must be regularly tested. For biological indicator verification points within the chamber, sterilization effectiveness should be verified quarterly using Bacillus stearothermophilus to ensure a sterility assurance level of 10^-6. Equipment maintenance requires the establishment of a complete electronic record system to automatically record each sterilization parameter, maintenance content, and calibration data. The data retention period must not be less than one year after the product's expiration date. Operators must undergo rigorous job training and be proficient in equipment operation, fault code identification, and emergency response procedures. When the equipment is out of use for a long time, the water in each pipe should be completely drained, the metal parts should be treated with rust prevention, and a complete performance confirmation should be carried out before reactivation. A scientific preventive maintenance system can not only extend the service life of the equipment, but also ensure the sterilization quality of each batch of products, providing a solid guarantee for drug safety. In the event of a control system failure or parameter abnormality, the deviation handling procedure should be immediately initiated, and the quality department and equipment engineers should jointly assess the impact and take corrective measures.



 English
English Español
Español Français
Français






















 CONTACT US
CONTACT US